News Items
- 05-Feb-2012
"ALSG approved Safe Transfer & Retrieval (STaR) course, Belfast - November 08th & 09th 2012" - 09-Oct-2011
"Survival-linX Solutions on-line Classrom & Education Centre" - 24-Sep-2011
"RIDDOR - Changes to incident reporting" - 12-Mar-2011
"Do you know what to do if a child is choking?" - 12-Mar-2011
"Anaphylaxis - recognition and management" - 12-Mar-2011
"Join use on Facebook - for all the latest discusions, first aid advice and general resuscitation information" - 12-Mar-2011
"Does your Surgery need Life Support (CPR) & Automated Defibrillator (AED) Training?" - 12-Mar-2011
"Resuscitation Council (UK) Emergency Life Support in Schools" - 04-Nov-2010
"2010 Resuscitation Guidelines - Summary of main changes" - 07-Sep-2010
"Do you have a Automated Defibrillator in your place off work?" - 24-Nov-2009
"Survival-linX Solutions gains HSENI certificate of approval to provide first aid at work (FAW) training courses." - 07-Nov-2009
"Risks of pandemic H1N1 2009 influenza (swine influenza) during cardiopulmonary resuscitation (CPR)" - 19-Oct-2008
"Are you thinking of purchasing an Automated External Defibrillator(AED) - essential infomation to assist you when choosing the correct unit." - 09-Aug-2008
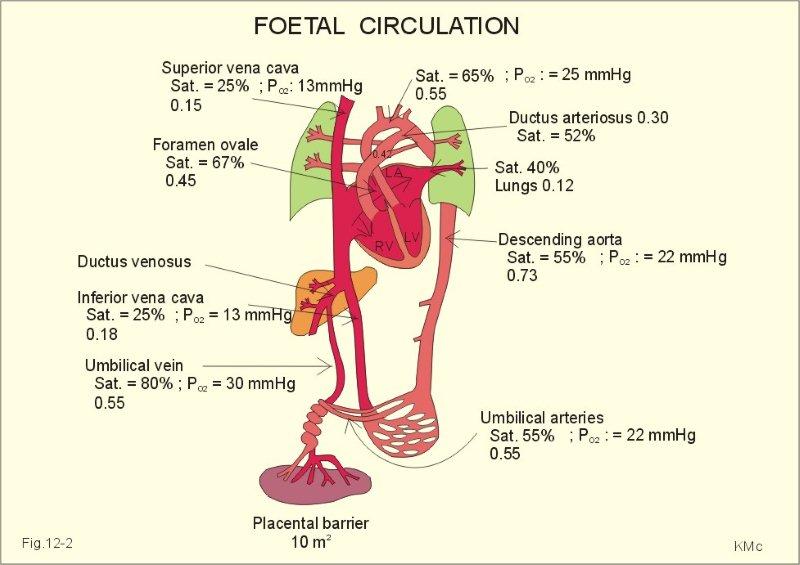
"An account of the formation of the foramen ovale and how blood entering the inferior vena cava receives preferential blood streaming in the foetus." - 31-Aug-2007
"Mechanical CPR - Lund University Cardiopulmonary Assist System (LUCAS)" - 02-Jun-2003
"Significant increase in training for Health Care professionals likely to be called to assist children with trachestomy tube difficulties"
An account of the formation of the foramen ovale and how blood entering the inferior vena cava receives preferential blood streaming in the foetus.
09-Aug-2008
The human cardiovascular system is complex and consists of a four chambered heart (pump) with extensive pathways of blood vessels (arteries and veins) and a separate lymphatic vascular system to distribute and collect blood from every part of the human body. The human heart is the first organ to form during embryogenesis (Zaffran and Frasch, 2002). Knowledge of cardiac embryology and the fetal heart permits an understanding of how heart formation can go wrong producing congenital abnormalities which are the most common of congenital defects (Sweeney 1998).
In foetal circulation the patent foramen ovale is necessary to permit highly oxygenated blood from the inferior vena cava to cross from the right to the left atrium thus bypassing the dormant pulmonary system. While other heart components are also formed during fetal development this assignment will analyse current literature to demonstrate a comprehensive understanding of the formation of the foramen ovale.
This literature review looks at what is known and what remains controversial and will concentrate on identifying how highly oxygenated blood and nutrients entering the inferior vena cava receive preferential streaming through the foramen ovale. As the development of the foramen ovale takes place in several stages these will be described in detail after the literature review.
Literature review – preferential blood streaming through the foramen ovale during fetal life.
The requirement of the fetus to receive placental transfusion which provides both oxygenated blood and nutrients (without passing through the lungs) to permit fetal life requires a cardiovascular system distinct from the postnatal situation (Schmidt et al 1996). In the developing fetus one such distinction is preferential streaming of blood through an opening between the right and left atrium (foramen ovale) thus bypassing the non functional pulmonary system (Lilly 2007). Streaming occurs as a result of blood with different oxygen and nutrient values travelling in the same vessel without mixing. This literature review will concentrate on identifying how highly oxygenated blood and nutrients entering the inferior vena cava receive preferential streaming through the foramen ovale to the left atrium which in turn supplies the heart and brain.
Reuss et al (1981) conducted forty experiments in sheep using radionuclide-labelled microspheres demonstrating preferential streaming from the umbilical veins (highly oxygenated blood) through the foramen ovale to the left atrium. Reuss et al (1981) also demonstrated that during hypoxia in the fetal sheep oxygen levels to the brain were maintained and increased to the myocardium. In comparison to other studies Reuss et al (1981) used a methodological design that was more rigorous by collecting data with the animals in ‘real life’ conditions. Monitoring catheters had been placed two days previously and the study was carried out postoperatively as the animal stood quietly in her cage. This element of the study demonstrated that the outcomes measured were objectively validated as each animal was free of anaesthetic agents and stress that may have altered the findings (Edelstone, 1978).
The protocol used by Reuss et al (1981) had however methodological limitations - manual injection of microspheres was employed making it highly likely that the intensity and circulation of injection rate (specific intervention) varied from study to study. Saura et al (2006) suggests studies should carefully control intensity and circulation of rate of contrast injected. This element questions the reliability and validity of the Reuss et al (1981) study design and if repeated perhaps the same results may not be obtained - mechanical infusion may have eliminated this problem. Consideration should also be given to potential turbulence caused by the rapid injection of microspheres into the umbilical blood flow which may have lead to unrealistic mixing of blood streams. The use of microspheres is recognised by Reus et al (1981) as a method that may overestimate the effects of streaming and this combined with varying placement of the monitoring catheter proximity to the heart leading to microspheres reaching the heart at different time intervals creates sufficient doubt in the findings of this paper. Interestingly confidence intervals were not reported which would have strengthened the evidence produced by Reuss et al (1981).
Macdonald et al (1988) studied the hearts of foetal and neonatal foals using scanning electron microscopy. In the foetal foal Macdonald et al (1988) observed the presence of a tubular structure constituting a ‘tunnel’ consisting of a ridge between the prominent crista dividens and atria tissue associated with the caudal vena cava. This observation supported observations made by a number of previous studies (Franklin et al 1942; Amoroso et al 1942; Ottaway 1944) confirming what was already known about this structure. Macdonald et al (1988) observed that this tubular structure only existed in the foetal foal and had collapsed in the heart of the newborn foal.
Macdonald et al (1988) further observed coagulated blood in the caudal vena cava suggesting the role this atrial tissue had in providing preferential streaming of highly oxygenated blood from the caudal vena cava across the foramen ovale. While their suggestion may be true other studies (Silver and Comline 1972, Barnes et al 1979) concluded that all blood in the foetal foal must pass through the liver (unlike the human foetus they do not have a ductus venous) leading to a decrease in inferior vena cava blood oxygenation. More importantly a decrease in caudal vena cava pressure due to blood flow through the liver could increase the possibility of placental and lower body blood mixing. For this reason blood in the foetal foal is less divided than that of a human embryo as it enters the right atrium from the inferior vena cava, an aspect of the study design that questions if this study population can be representative of or generalised to the human fetus.
In a later study using foetal sheep Klaus et al (1996) used multimodal ultrasound to assess blood flow and blood velocity from the intra-abdominal part of the umbilical vein, the caudal vena cava and subsequently through the foramen ovale. In all animals studied two blood streams (ductus venous and caudal inferior vena cava) were identified originating from within the inferior vena cava (IVC) showing consistency with the previous MacDonald 1988 study. Klaus et al (1996) demonstrated with contrast echocardiograms and doppler colour flow that these two blood streams were very distinct. Analysis also showed that most of the blood entering from the superior vena cava (blood low in oxygen) was directed (streamed) to the right ventricle.
Using echocardiogram with saline Klaus et al (1996) observed a higher proportion (although not quantified) of blood streaming directed from the ductus venosus (highly oxygenated blood) through the foramen ovale vs. blood streaming from the caudal vena cava (poorly oxygenated blood) which flowed into the right ventricle. Klaus et al (1996) suggests a circumferential constriction in the ductus venous as accounting for the creation of high velocity blood flow although this is only evidenced by doppler velocity and not observed microscopically. The high velocity of the ductus venous flow was noted compared with the caudal inferior vena cava velocity (mean velocity 57 +/- 13 versus 16+/- 2cm/s). While the results were statistically significant (p < .0002) one would have to question the small population sample (n=8) and more work with a larger sample may be required in this area.
Explanation for the high velocity of ductus venous blood is unclear with other options such as a narrow ductus venous or the presence of a contractile sphincter which has been noted in fetal lambs (Barron 1942) other possible answers.
The ability to assess differing flow velocities in real time and specific identification of blood flow origin through use of saline contrast injections in the Klaus et al (1996) study was a significant advancement. This method does however have limitations as it does not provide specific quantitation of flow a fact that limits this study in reaching definitive conclusions. Catheter position in the vessel is not clearly stated and may have varied from animal to animal raising questions regarding the use of mean velocity recordings. Recognition that varying positions in the catheter position may account for changes in ductus velocity recordings unrelated to changes in blood flow are stated. This important factor in the study design protocol questions the stability of the measurement tool which in turn may have affected reliability and study validly by producing erroneous results.
Polit and Beck (2006) suggest that the overriding criteria in sampling is to what extent the sample is similar to the population being studied. Klaus et al (1996) studied foetuses that were anaesthetised and had operations performed. These interventions performed for ethical and humanitarian reasons are known to affect blood flow (Edelstone, 1978). This fact prevents Klaus et al (1996) conclusions from being generalised as these conditions are not representative of that of the human fetus. Caution in interrupting these findings is required as use of animal models and extrapolation of results to humans carries risks and is liable to be invalid (Greenhalgh 2006).
Research limitations, implications & conclusions.
What is verified from animal experiments is that there is preferential streaming of highly oxygenated blood to the brain and upper body however controversy exists with regard to how blood streaming happens. Klaus et al (1996) postulated that a valve deflected ductus venous blood towards the foramen ovale, Kiserud et al (1992) related blood streaming to velocity and Macdonald et al (1988) observed the presence of a tubular structure constituting a ‘tunnel’. The evidence concludes that selective streaming of oxygenated and deoxygenated blood exists however the mechanisms whereby oxygenated blood is directed through the foramen ovale has not been clearly defined. Understanding of fetal physiology is largely based on experimental studies in animals and results of post-mortem due to the inaccessibility of the human foetus in situ and suitable methods for foetal circulatory examination before birth. Current literature does not develop specific evidence for use in clinical practice as the human fetus having similarity with animal physiology also has some important differences.
Development of more modern techniques in particular echocardiogram should permit future studies to investigate under physiological conditions the mechanism which provides preferential streaming of oxygenated blood through the foramen ovale and strengthen evidence that currently exists. Whatever the evidence foetal and newborn blood flow must achieve the same functions – deliver oxygenated blood and nutrients to the major organs and return the carbon dioxide to the gas exchange organ to allow gas exchange.
Cardiac Embryology
Septum primum and foramen primum
The appearance of the vascular system begins to appear in the third week of gestation when nutritional requirements of the embryo are no longer satisfied by diffusion alone within the yolk sac (Sadler 2006). A horse-shaped endothelial-lined tube a region known as the cardiogenic field is formed through uniting blood islands and cardiac myoblasts a process known as vasculogenesis. Around day nineteen (Larsen 1997) of cardiac embryology there consists bilateral endocardial and myocardial tubes (heart tubes) formed from the cardiogenic field eventually fusing at day twenty two (England 1996). In doing so heart structures/regions are formed within one expanded heart tube (Sweeney 1998) consisting of an inner endothelial lining and outer myocardial layer. Specific derivates of each region of the heart tube include,
• Sinus Venous
• Primitive atrium
• The Atrioventricular canal
• The primitive ventricle
• Bulbus cordis
• Truncus arteriosus
By the end of the fourth week the heart tube begins to beat (pump) in a systematic fashion from atrium to ventricle (Sadler 2006) and circulate blood cells in a unidirectional flow throughout the embryo.
Restriction, obstruction, premature closure or non formation of the foramen ovale is associated with serious cardiac failure and fetal death (Hagen et al 2005). Dramatic reorganisation of the circulatory system is required during the transition from fetal to extrauterine life with the neonatal circulation adapting gradually.
Formation of the cardiac loop, systemic and pulmonary circulation.
As the heart tube continues to develop and elongate rapidly in length during intrauterine life two umbilical veins (right and left) open into the venous (caudal end of the heart tube) while two primitive aorta forms from the cephalic end (arterial). The elongated heart tube begins to loop and fold bringing the future chambers of the heart into correct spatial relation to each other (Larsen 1997) forming three septa - the atria, great veins and ventricle.
As the developing fetal heart continues to develop and remodel the lungs remain collapsed while the pulmonary system is non functional. Continual placental blood flow is required to bring oxygen and nutrients from the mother to the developing embryo with an increase in blood flow with fetal gestation (Link et al 2007). Over time the right umbilical vein is obliterated leaving the left umbilical vein which forms a channel called ductus venous (vein between the liver and heart) with the inferior vena cava shunting
blood to the right atrium. Umbilical arteries return the waste products to the maternal circulation via the placenta.
Development of interatrial membranes.
Numerous studies (Rogers C. 1986, Morse D. 1986, Arrechedera H. 1998) have demonstrated the interatrial thin membranous septum mesenchymal tissue (septum primum) originating from the dorsocranial of the primitive atrium wall. The septum primum advances across the common atrium towards the endocardial cushions (atria – ventricular junction) which divide the atrium from the ventricle. The septum primum constricts the foramen between the atrial chambers forming the ostium primum which permits blood shunting across the partitioned right and left atrium (Larsen 1997). Apoptosis (programmed cell death) creating a new orifice (ostium secundum) develops high in the interatrial septum. The ostium primum closes only after the ostium secundum enlarges to permit right to left shunting of blood which persists throughout gestation until birth.
The ostium secundum allows the uninterrupted flow of blood from the right atrium into the left atrium (Amaral 2007). Another thick and muscular (compared to the septum primum) crescent shaped interatrial septum (septum secundum) begins to develop extending down over the ostium secundum however never forms a complete partition(Sadler 2006) but a unidirectional valve permitting blood to flow only from the right to the left atrium. The septum secundum contains a permanent opening which forms the foramen ovale.
The upper part of the septum primum gradually degenerates with the lower part remaining to cover the foramen ovale forming a one way valve (valve of the foramen ovale) that opens under pressure to permit blood to flow from the right to the left atrium only (England 1996). High right atrial pressure (compared with the left atrium) keeps the valve of the foramen ovale open allowing highly oxygenated fetal blood to move across the heart bypassing the pulmonary system (Appendix 2).
Circulation after birth
At birth left atrium pressure increases above right atrium pressure (Sun 1996) forcing the valve of the foramen ovale to close against the septum secondum. Anatomically fibrous adhesions develop to create a permanent seal closing the interatrial septum (Hagan 1984) during the first year of fetal life. The closing of the foramen ovale diverts all blood returning to the right hand side of the heart to the pulmonary system which removes carbon dioxide and oxygenates the blood which then returns to the left side of the heart for distribution to the tissues. In approximately 25 – 30% of cases a permanent seal is not complete and a patient foramen ovale persists (Hagan 1984). Clinical problems for example stroke (Lechat 1988), migraine (Azarbal 2005), decompression illness (Torti 2004) and platypnoea-orthodeoxya (Cheng 2000) are associated with a patent foramen ovale.
References
Amaral, H., 2007. Morphological basis for the study of the interatrial septum in the human fetus. Arquivos Brasileiros de Cardiologia. 88. (5). pp.559 – 564.
Amoroso, E., Barclay, S., Franklin, K. and Prichard, M., 1942. The bifurcation of the posterior caval channel in the eutherian foetal heart. Journal Anatomy. 74. pp. 240 – 247.
Arrechedera, H., Alvarez, M., Strauss, M and Arguello, C., 1998. Myocardial interatrial septum loses its epithelial organization by mesenchymal influence structural and ultrastructural study. Journal of Submicroscopic Cytology. 30. (1). pp. 95 – 103.
Azarbal, B., Tobis, J., Suh, W., Chan, V., Dao, C. and Gastor, R., 2005. Association of interatrial shunts and migraine headaches: impact of transcatheter closure. Journal American College Cardiology. 45. (4) pp 489 – 492.
Bahlmann, F., Wellek. S., Reinhardt. I., Merz, E. and Welter, C., 2000. References values of ductus venosus flow velocities and calculated waveform indices. Prenatal Diagnosis. 20. pp 623 – 634.
Barclay, A., Barcroft, J., Barron, D. and Franklin, K., 1939. A radiographic demonstration of the circulation through the heart in the adult and in the fetus and the identification of the ductus arteriosus. British Journal Radiology. 12.pp 595.
Barnes, J., Comline, S., Dobson, A., Silver, M., Burton, G. and Steven, H., 1979. On the presence of a ductal venous in the fetal pig in late gestation. Journal of developing physiology. 1. pp.105 – 110.
Barron, D., 1942. The sphincter of the ductus venous. Anatomy Rec. 82. pp 398 (Abstract).
Cheng, T., 2000. Transcatheter closure of patent foramen ovale: a definitive treatment of platypnea-orthodeoxia. Catheter Cardiovascular Intervention. 51. (1) pp.120.
Edelstone, D., Rudolph, A. and Heymann, M., 1978. Liver and ductus venous blood flows in fetal lambs in utero. Circulation Research. 42. pp. 426 – 433.
Edelstone, D. and Rudolph, A., 1979. Preferential streaming of ductus venous blood to the brain and heart in fetal lambs. American Journal of Physiology. 237. H724 – H729.
England, M., 1996. Life before birth. 2nd Edition. London, Mosby-Wolfe.
Fink, F., 1998. Conducting research literature reviews. SAGE Publishing. London.
Franklin, J., Amorosa, E., Barclay, A., and Prichard, M., 1942. The valve of the foramen ovaleand its relation to pulmonary vein entries. Veterinary Journal. 98. pp. 29 – 41.
Greenhalgh, T., 2006. How to read a paper. BMJ Publishing Group. London.
Hagan, A, Albig, M, Schmitz, L, Van Baalen, A, Becker, R and Entezami, M., 2005. Prenatal Diagnosis of Isolated Foramen ovale Obstruction. Fetal Diagnosis and Therapy. 20, pp.70 – 73.
Hagan, P., Scholz, D and Edwards, W., 1984. Incidence and size of patent foramen ovale during the first 10 decades of life: an autopsy study of 965 normal hearts. Mayo Clinical Procedures. 59. pp 17 – 20.
Huisman, T., Stewart. P. and Wladimiroff. J., 1992. Ductus venous blood flow velocity waveforms in the human fetus – a doppler study. Ultrasound Medical Biology. 18. pp 33 – 37.
Kiserud, T., Eik-Nes, S., Hellevik, L. and Blaas, H., 1992. Ductus venous: a longitudinal Doppler velocimetric study of the human fetus. Journal Maternal Fetal Investigation. 2. pp.5-11.
Larsen, W., 1997. Human Embryology. 2nd Edition. United Kingdom. Churchill Livingstone.
Lechat, P., Mas, J., Lascault, G., Loron, P., Theard, M., Klimczac, M., 1988. Prevalence of patient foramen ovale in patients with stroke. New England Journal of Medicine. 318. (18) pp.1148 -1152
Lilly, L., 2007. Pathophysiology of Heart Disease. 4th Edition. USA. Lippincott Williams & Wilkins.
Link G., Clarke E. and Lang U., 2007. Umbilical blood flow during pregnancy; evidence for decreasing placental perfusion. American Journal of Obstetrics and Gynaecology. 196. (5), pp. 489 e1 – 489 e7.
Macdonald, A., Fowden, A., Silver, M., Ousey, J and Rossdale, P., 1988. The foramen ovale of the foetal and neonatal foal. Equine Veterinary Journal. 20. (4), pp. 255 – 260.
Morse, D., Rogers, C. and McCann, P., 1986. Atrial septation in the chick and rat a review. Journal of Submicroscopic Cytology. 16. (2). pp. 259 – 272.
Ottaway, C., 1944. The anatomical closure of the foramen ovale in the equine and bovine heart: A comparative study with observations on the foetal and adult states. Veterinary Journal. 100. pp 111 -118, 130 – 134.
Polit, D. and Beck, C.T., 2006. Essentials of Nursing Research Methods, Appraisal and Utilization. 6th Edition. Lippincott Williams. New York.
Sadler, T., 2006. Langman’s Essential – Medical Embryology. 10th Edition. United States. Lippincorr Williams and Wilkins.
Saura, D., Garcia-Alberola, A., Florenciano, R., de la Morena, G., Sanchez-Munoz, J.J., Soria, F., Martinez-Sanchez, J. and Valdes-Chavarri, M., 2007. Alternative explanations to the differences of femoral and brachial saline contrast injections for echocardiographic diction of patient foramen ovale. Medical Hypotheses. 68. pp. 1378 – 1381.
Rogers, C., 1986. Atrial septation in the rat. A light microscopic and histochemical study. Journal of Submicroscopic Cytology. 18. (2). pp. 313 – 324.
Rudolph, A. and Heymann, M., 1967. The circulation of the fetus in utero: methods for studying distribution of blood flow, cardiac output, and organ blood flow. Circulation Research. 1967. 21. pp. 163 – 184.
Schmidt, K., Silverman, N. and Rudolph, A., 1996. Assessment of Flow Events at the Ductus VenosusInferior Vena Cava Junction and at the Foramen Ovale in Fetal Sheep by Use of Multimodal Ultrasound. Circulation. 93. pp.826 – 833.
Silver, M. and Comline, S., 1972. Some observations on umbilical circulation of the foal compared with the ruminant. In: Respiratory gas exchange and blood flows in the placenta. US Department of Health. pp 113 – 115.
Sun, J., Stewart, W., Hanna, J. and Thomas, J., 1996. Diagnosis of patient foramen ovale by contrast versus color Doppler by transeophageal echocardiography: Relation to atrial size. American Heart Journal. pp. 239 – 243.
Sweeney L., 1998. Basic Concepts in embryology: A student’s survival guide. United States. McGraw-Hill.
Torti, S., Billinger, M., Schwerzmann, M., Vogel, R., Zbinden, R. and Windecker S., 2004. Risk of decompression illness among 230 divers in relation to the presence and size of patent foramen ovale. European Heart Journal. 25 (12) pp 1014 – 1020.
Zaffran, S. and Frasch, M., 2002. Early signals in cardiac development. Circulation. 91. pp 457 - 469
Register
If you do not have access please register here to access our members section. If you have forgotten your password please click here to reset your password.
Contact Us
| Address | PO Box 1491 Dungannon BT71 5YF |
| Telephone: | 028 8774 6864 |
| Mobile: | 079 7662 1643 |
| Email: | info@survival-linx.com |